The current Brazilian government has been prolific in atypical practices since its coming to power in 2019. The handling of issues related to the current COVID-19 pandemic is no exception. This post addresses recent government announcements related to the pandemic and the ensuing reactions it arose.
Our first post to this blog concentrated on measures attempted by the Health Ministry related to the disclosure of pandemic data. The post described the situation and concerns raised in response to the government’s attempt – an attempt that was not implemented after all.
Despite many other unusual measures by the Health Ministry, this post concentrates on a move made by another Brazilian Ministry, the Science, Technology and Innovation Ministry (MCTI). The current minister heading MCTI is an air force pilot who became famous after being the only Brazilian to go into space, as part of a single 10-day Russian mission some 15 years ago.
In 19 October 2020, the MCTI held a ceremony attended by the country’s president and many state ministers to announce that a study supported by the Ministry proved that the drug Nitazoxanide, a vermifuge for worm treatment, was effective for early treatment of COVID-19. The claim was based on a study carried out by a team led by Dr Patricia Rocco, a well-established and competent scientist working in a Biophysics laboratory at Universidade Federal do Rio de Janeiro.
The MCTI announcement caught the attention of the Brazilian society and members of academia for a number of reasons. First, the MCTI has a number of research supporting calls that enable many research projects, including my own. A large number of projects undertaken in 2020 were concerned with various aspects of COVID-19 but none of them was given an individualized public announcement like this one. Also, the announcement did not provide any detail about the experiment. The explanation was that the release of the results would remove the novelty of the experiment and would hinder its scientific publication.
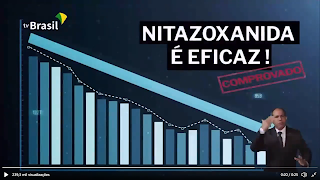
Screen shot of the video of the ceremony taken at about 20:40.
Another striking feature of the announcement was the use of an image borrowed from the image databank Shutterstock. This was done without proper acknowledgment, a basic practice in rights recognition. Use of the image could have led the general public to believe the image had a connection with the study. It also raised questions about the seriousness of the MCTI, leaving an impression of amateurism in such an important and relevant issue that demands the most professional treatment possible. The image above is a screenshot taken from the official government video showing the aforementioned image with text “Nitazoxanide is effective; proven” superimposed by the government.
The lack of details provided about the study in the announcement received country-wide criticism from scientists. After 4 days, a pre-print was published in MedRxiv and details became clearer. It described an apparently well conducted experiment with more than a thousand patients but only a few hundred of those patients were included in the statistical analyses. The evidence was summarized in favour of the drug based on the p-values obtained from statistical tests comparing drug and placebo groups. The main results reported as statistically significant were obtained for percent viral load reduction in the 2 arms; and p-values ranged from 0.006 to 0.013, while results for other outcomes, such as symptom resolution, were not found to be statistically significant.
A fair number of criticisms still remained after evaluating the results relative to the design and analysis. Many concerns were raised, especially by the government’s conclusion of “proof” based on a single, limited study. Demands for more studies to evaluate the evidence indicated by this study were voiced. A summary of some of them was published in a Science magazine issue. We are not aware of responses to the concerns, but the paper describing the study and its results was finally accepted for publication in a European journal a few months later (full version here). There was no surprise when the MCTI once again made an official announcement about this publication as if a scientific seal of approval was granted to the use of this drug for the so-called “early treatment of COVID-19.”
This history is particularly important given the stated commitment of the Brazilian federal government to provide early treatment for COVID-19, despite criticisms. It started with chloroquine, then moved to invermectine and then introduced nitazoxanide. All were accompanied by sizeable public investments in marketing, purchasing, and production that are all doomed to be wasted or, even worse, potentially harmful.
Science does not progress this way. Every piece of evidence should be weighed against existing knowledge and other pieces of evidence. This is how Science is built and has evolved over millenia. It is not uncommon to find results supporting a hypothesis that were restricted to the specific conditions under which they were obtained and were later discredited by further studies.
Attempts to short-cut the scientific process could be harmful not only to scientific progress and to trust in Science, but also to the public that will be the users of the scientific discoveries. The Brazilian government seems to be having a hard time understanding this. We all wish for a positive answer to the question posed as the title to this post, but so far the only scientifically certified weapons against this disease are sanitation, mask wearing, social distancing and, more recently, immunization by the vaccine.